For Life-Science Companies Ready to Leave Validation Bureaucracy Behind
You Run the Business. We Run the Validation.
Full-service delivery of your CSV/CSA projects — with structure, speed, and compliance seamlessly built in.
- Audit-ready results, delivered by embedded experts
- Fully managed execution across planning, testing & reporting
- Risk-based, lean validation – tailored to your environment
- Cross-functional coordination from start to signoff
- 15+ years of experience in pharma, biotech & MedTech systems
Delegate your validation. Stay focused on operations.



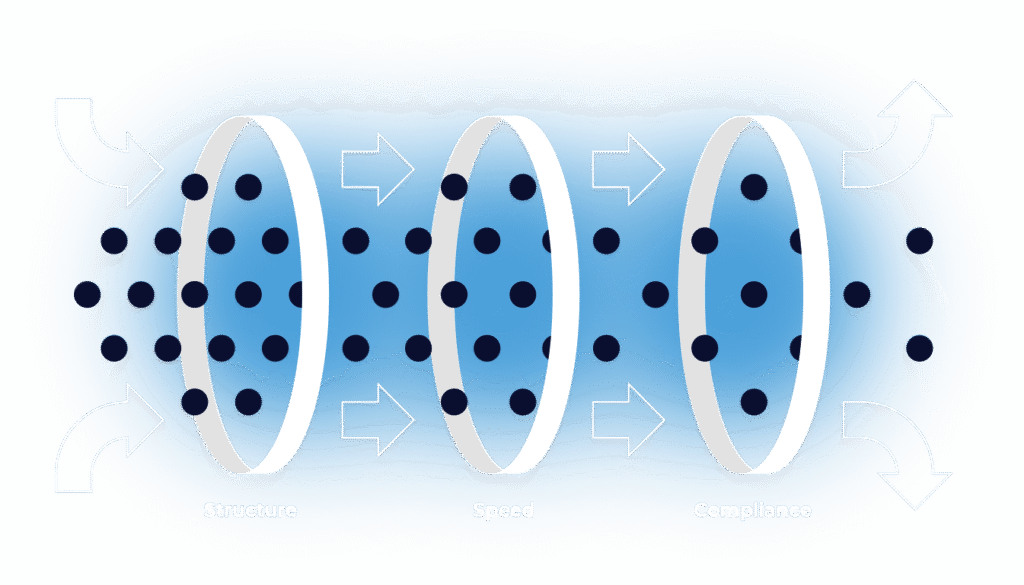
Validation Isn't Hard. Running it Without Structure Is.
- Everyone thinks someone else is in charge
- SOPs create more confusion than clarity
- Timelines slip in slow-motion
- Testing gets blocked by uncoordinated reviews
- QA and IT speak different languages
- Business gets tired of hearing about validation delays
You don’t need support. You need delivery.
Validation Delivered with Clarity,
Speed, and Total Ownership

Rapid Onboarding & Project Start
We jump in fast. No waiting, no warm-ups. Alignment, planning, and mobilization in days, not weeks.

End-to-End Ownership
From risk planning and documentation to testing, deviations, and audit prep—we manage everything.
Built Around Your Environment
We adapt to your systems, templates, and tools. No forced frameworks.
No disruptions.
Audit-Ready Handover
Every document. Every decision. Fully traceable, structured, and compliant. Delivered as a clean, inspection-ready package.
Your Done-for-You Validation Execution Includes:
Validation Leadership & Stakeholder Coordination
Planning & Documentation
Testing & Execution
Lean Governance & Methodology
Risk-based, scalable, and adaptable — ready for any system, any team.
Real Team
Enablement
We coach, support, and equip your internal experts to own the process long-term.
Daniel Herrmann Consulting
- 60+ systems delivered in 8+ countries
- Audit-tested, real-world frameworks trusted by Top 30 pharma and scale-ups alike
- Specialists in CSV, CSA, lean compliance, and digital validation governance
- German precision. Global execution.
Need validation delivery without the drama?
Client Success Stories
Boehringer Ingelheim – SAP/EWM Rollouts
Challenge: Complex international teams, tight deadlines
Result: Full validation delivery, audit-ready handover, on-time go-live
„Daniel didn’t just join the project — he made it succeed.“
Top-5 Pharma – ERP Program
Challenge: Fragmented roles, poor stakeholder alignment
Result: Integrated validation plan, unified execution, no audit issues
„He turned confusion into execution.“
Mid-Size Pharma – QMS Rollout
Challenge: Legacy SOP chaos, overworked QA
Result: Documentation streamlined, review flow rebuilt, successful audit
„We could finally breathe again. The system worked — and so did we.“
*Testimonials have been anonymized to comply with data protection regulations.
Take Action
Full delivery from planning to final report
Clean documentation, clear roles, no delays
Compliance without overload, execution without compromise
We answer the tough questions upfront:
Do we stay involved?
Yes. You stay informed, empowered, and in control. But we handle the execution and delivery.
Do you work with our tools and SOPs?
Absolutely. We adapt to your environment, not the other way around.
What systems have you validated?
ERP (SAP S/4HANA, EWM), LIMS, Veeva QMS, DocuSign integrations, custom SaaS, and cloud systems.
What makes this different from typical CSV support?
We don’t just advise. We own. We drive validation forward and deliver results.
Can we scale this across multiple sites?
Yes. We’ve delivered multinational programs and align easily with global governance models.
Still have questions? Let’s find the answers together. Book your call now.
Daniel Herrmann Consulting
Saarlouiser Straße 95
66740 Saarlouis

