For Life-Science Companies Ready to Leave the nonsense bureaucracy of CSV and IT-Processes behind
The IT-compliance boutique you've been looking for — lean, audit-safe, and built for execution.
- Cut your CSV and operational IT costs by up to 30% and timeline by 50% with our Lean IT-Compliance System
- Build audit-ready systems that scale with your growth.
- Complete validation projects without overloading your internal teams.
- Empower your team with expert monitoring and lean CSA-aligned methods
- Gain senior-level validation leadership — without adding headcount.




It’s not your fault. The industry made it this way.
But the cost of staying stuck are high. Missing deadlines, audit risks, and wasted resources.
Validation today is slow, overly bureaucratic, and disconnected from operational reality. Similarly, IT compliance as a whole strains considerably more capacity than necessary.
The old methods?
They were built to drown teams in paperwork, not drive real outcomes.
And the big consulting firms? They just kept piling on templates and checklists — keeping the system broken.
And while regulators introduced CSA and opened the door to leaner approaches,
much of the industry stayed stuck, married to the bloated ways of the past.

Here’s the bottom line:
IT-Compliance, Reengineered
for Modern Life-Science Operations

Accelerated Execution

Audit-Proven Outcomes
Lean Governance & Methodology
Real Team Enablement
Validation, Reengineered for
Modern Life-Science Operations
Lean IT-Compliance
Project Validation Execution (Done-for-You)
You Run the Business. We Run the Validation.
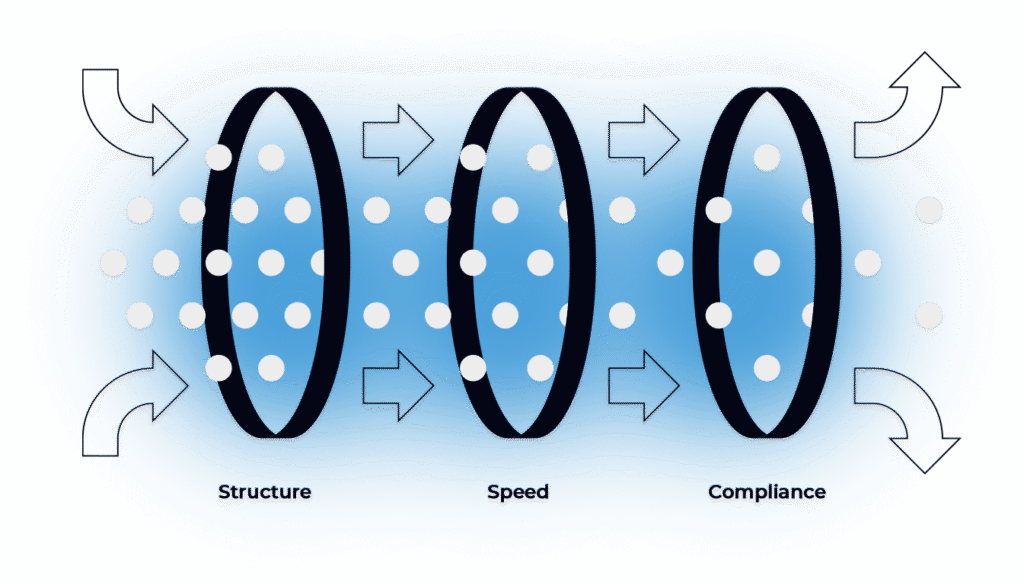
Full-service delivery of your CSV/CSA projects — with structure, speed, and compliance seamlessly built in
Project Validation Coaching (Done-with-You)

Remote Validation Management Organization

Program Methodology for Large-Scale Rollouts

Daniel Herrmann Consulting
We don’t just validate systems.
We build validation systems that drive speed, clarity, and control — across ERP, QMS, LIMS, and custom environments.
- 60+ systems delivered in 8+ countries
- Audit-tested, real-world frameworks trusted by Top 30 pharma and scale-ups alike
- Specialists in CSV, CSA, lean compliance, and digital validation governance
- German precision. Global execution.
Join the innovators transforming validation into a growth driver.

Client Success Stories
Mid-Sized Pharma Company – Global ERP Validation
Result: Validation timelines reduced by 35%. ERP Go-Live on-time. Zero critical audit findings.
“Daniel turned our biggest bottleneck into an operational win.”
Top-10 Pharma Company – GxP System Integration
Problem: Long validation delays and overwhelmed internal teams put critical system launches at risk.
Result: 20+ go-lives in 12 months. 40% reduction in internal CSV workload.
“No one knows execution like Daniel Herrmann Consulting. Period.”
Biotech Scale-Up – eQMS Validation
Problem: No scalable validation methodology. Overwhelmed QA teams at risk of FDA non-compliance.
Result: Zero FDA findings. eQMS live in 10 weeks.
“Our future is built on the foundation Daniel gave us.”
*Testimonials have been anonymized to comply with data protection regulations.
Take Action
We answer the tough questions upfront:
What makes the Lean IT-Compliance system audit-safe?
How fast can you start?
What results can I expect?
Can you work with our existing processes?
Still have questions? Let’s find the answers together. Book your call now.
Daniel Herrmann Consulting
Saarlouiser Straße 95
66740 Saarlouis

